Crystallographic and electronic evolution of lanthanum strontium ferrite (La0.6Sr0.4FeO3−δ) thin film and bulk model systems during iron exsolution
Abstract
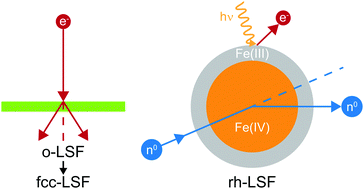
We study the changes in the crystallographic phases and in the chemical states during the iron exsolution process of lanthanum strontium ferrite (LSF, La0.6Sr0.4FeO3−δ). By using thin films of orthorhombic LSF, grown epitaxially on NaCl(001) and rhombohedral LSF powder, the materials gap is bridged. The orthorhombic material transforms into a fluorite structure after the exsolution has begun, which further hinders this process. For the powder material, by a combination of in situ core level spectroscopy and ex situ neutron diffraction, we could directly highlight differences in the Fe chemical nature between surface and bulk: whereas the bulk contains Fe(IV) in the fully oxidized state, the surface spectra can be described perfectly by the sole presence of Fe(III). We also present corresponding magnetic and oxygen vacancy concentration data of reduced rhombohedral LSF that did not undergo a phase transformation to the cubic perovskite system based on neutron diffraction data.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...