Cyclization reaction dynamics of an inverse type diarylethene derivative as revealed by time-resolved absorption and fluorescence spectroscopies†
Abstract
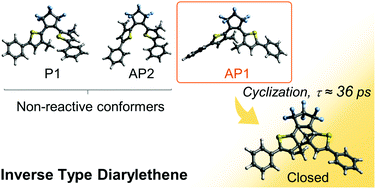
Photocyclization reaction dynamics of an inverse type diarylethene derivative was investigated in alkane solutions by means of ultrafast laser spectroscopies. Femtosecond transient absorption spectroscopy showed that the Franck–Condon state formed by photoexcitation is geometrically relaxed to a transient species within 100 fs and subsequently the cyclization process takes place with a time constant of 36 ps. This time constant is much longer than those in normal type derivatives. Steady-state and time-resolved fluorescence measurements with the aid of quantum chemical calculations revealed that there exist three kinds of conformers, one parallel and two anti-parallel forms, in the ground state. One of the anti-parallel conformers undergoes the cyclization reaction, while the other two conformers are nonreactive species and their major relaxation processes are radiative decay and intersystem crossing into the triplet states. The triplet states thus formed no longer undergo the cyclization reaction in the late time region.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...