Effects of interfacial specific cations and water molarities on AOT micelle-to-vesicle transitions by chemical trapping: the specific ion-pair/hydration model†
Abstract
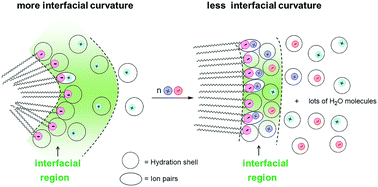
Salt induced micelle-to-vesicle transitions of ionic surfactants depend on the surfactant chain length, headgroup structure, counterion type and concentration, but the interfacial molarities of counterions and water that balance the hydrophobic effect are difficult to determine. In anionic micelles of twin-tailed sodium bis(2-ethylhexyl)sulfosuccinate (AOT), the chemical trapping (CT) method provides estimates of the interfacial molarities of anionic headgroups (RSO3−m) and neutral (H2Om) nucleophiles during salt induced transitions of AOT micelles to vesicles. Product yields were measured by HPLC from the competitive dediazoniation reaction using a specially designed hydrophobic probe, 4-hexadecyl-2,6-dimethylbenzenediazonium cation, 16-ArN2+. The reactions were run at constant concentration of 15 mM AOT mixed with 0 to 50 mM added salts, containing cations of different sizes and valences including tetraalkylammonium cations (MR4+, R = 1–4) and metal cations (M1–3+). Parallel reactions in aqueous salt solutions with a short chain analog, 1-ArN2+, were used as references to calculate interfacial molarities. Aggregates were structurally characterized by TEM and DLS. Typically, interfacial RSO3− molarities increase with added salts from 1 to 2 M and water molarities decrease from about 40 to 20 M with the micelle to vesicle transition. These changes are consistent with the ion-pair/hydration model, in which the added cations form neutral but polar ion-pairs with RSO3− that have a lower demand for hydration and water was released into the surrounding aqueous phase. The extent of ion-pairing increases with cation size, charge and hydrophobicity and decreases with interfacial water molarity, which permits tighter interfacial packing and vesicle formation at lower added salt concentrations.


 Please wait while we load your content...
Please wait while we load your content...