Multi-electron reduction of Wells–Dawson polyoxometalate films onto metallic, semiconducting and dielectric substrates†
Abstract
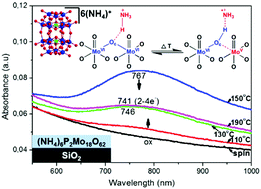
The investigation of conditions allowing multi-electron reduction and reoxidation of polyoxometalate (POM) films onto solid substrates is considered an issue of critical importance for their successful incorporation in electronic devices, different types of sensors and catalytic systems. In the present paper, the rich multi-electron redox chemistry of films of Wells–Dawson ammonium salts, namely (NH4)6P2Mo18O62 and (NH4)6P2W18O62, on top of metallic (Al), semiconducting (ITO) and dielectric (SiO2) substrates under ambient conditions is investigated. The respective Keggin heteropolyacids, H3PMo12O40 and H3PW12O40, are also investigated for comparison. On Al substrates, the Wells–Dawson ammonium salts are found to be significantly more reduced (4–6e−) compared to the respective Keggin heteropolyacids (∼2e−), in accordance with their deeper lying lowest unoccupied molecular orbital (LUMO) level. Subsequent thermal treatment in air results in reoxidation of the initially highly reduced POM films. Similar behavior is found on ITO substrates, but in initially less reduced (2–4e−) Wells–Dawson POM films. On the other hand, on SiO2 substrates, the thermal reduction of (NH4)6P2Mo18O62 film is observed and attributed to the thermal oxidation of ammonium counterions by [P2Mo18O62]6− anions. Overall, the multi-electron reduction of Wells–Dawson ammonium salts onto metallic and semiconducting substrates (Al, ITO) is determined by the relative position of the LUMO level of POMs in relation to the Fermi level of the substrate (i.e. substrate work function) and affected in a synergistic way by the presence of ammonium counterions. In contrast, on dielectric substrates (SiO2) the reduction of Wells–Dawson POMs ((NH4)6P2Mo18O62) is attributed only to the oxidation of ammonium counterions.


 Please wait while we load your content...
Please wait while we load your content...