Key factor governing the physicochemical properties and extent of proton transfer in protic ionic liquids: ΔpKa or chemical structure?†
Abstract
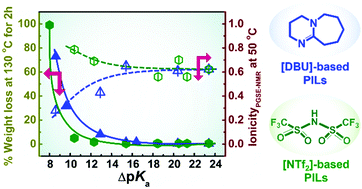
In order to identify the key factor governing the transport properties and extent of proton transfer in protic ionic liquids (PILs), a series of PILs were prepared by simple neutralisation of a super-strong acid, bis(trifluoromethanesulfonyl)amide acid (H[NTf2]), with a range of amines comprising diverse structures, including secondary and tertiary amines. The bulk physicochemical properties of the resulting PILs with a wide variation in the ΔpKa values of the constituent acid and protonated bases were compared to those of previously reported PILs derived from a super-strong base, 1,8-diazabicyclo[5.4.0]-7-undecene (DBU), and different acids (M. S. Miran, H. Kinoshita, T. Yasuda, M. A. B. H. Susan and M. Watanabe, Phys. Chem. Chem. Phys., 2012, 14, 5178–5186) with emphasis on the thermal stability, vibrational spectroscopy, density, viscosity, conductivity, and ionicity characteristics. The thermal stability of all PILs, as analysed by thermogravimetric analysis (TGA), was found to linearly increase with the ΔpKa values. However, isothermal TGA measurements for an extended duration indicated that [NTf2]-based PILs exhibit higher thermal stability than [DBU]-based PILs at low ΔpKa values (<15). PILs with secondary amines showed higher viscosity and lower ionic conductivity than those with tertiary amines because of the formation of multiple hydrogen bonds. The evaluated ionicity suggested that all [NTf2]-based PILs are “good PILs”, irrespective of the basicity of the amines, and have higher ionicity than [DBU]-based PILs particularly at low ΔpKa values (<15).


 Please wait while we load your content...
Please wait while we load your content...