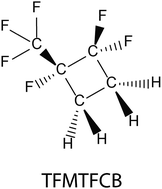
Atmospheric chemistry of a cyclic hydro-fluoro-carbon: kinetics and mechanisms of gas-phase reactions of 1-trifluoromethyl-1,2,2-trifluorocyclobutane with Cl atoms, OH radicals, and O3
Abstract
Long path length FTIR-smog chamber techniques were used to study the title reactions in 700 Torr of N2 or air diluent at 296 ± 2 K. Values of k(Cl + 1-trifluoromethyl-1,2,2-trifluorocyclobutane (TFMTFCB)) = (1.16 ± 0.21) × 10−14 and k(OH + TFMTFCB) = (3.51 ± 0.88) × 10−14 cm3 molecule−1 s−1 were measured. No reactivity of TFMTFCB towards ozone was observed. The atmospheric lifetime of TFMTFCB is determined by the reaction with OH and is approximately 330 days. The chlorine initiated oxidation gives C(O)F2 and CF3C(O)F as the dominant products in yields of (92 ± 2)% and (89 ± 2)%, respectively. The OH radical initiated oxidation gives C(O)F2 and CF3C(O)F as the dominant products in yields of (91 ± 6)% and (84 ± 4)%, respectively. The GWP100 was calculated as 44. The atmospheric chemistry of the title compound, a cyclic halogenated alkane, is discussed in the context of other halogenated cyclo-alkanes.


 Please wait while we load your content...
Please wait while we load your content...