Why co-catalyst-loaded rutile facilitates photocatalytic hydrogen evolution†
Abstract
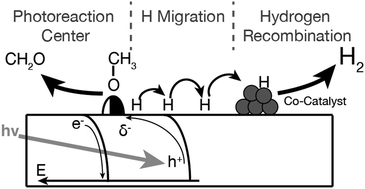
As the conduction band edge of rutile is close to the reduction potential of hydrogen, there is a long-lasting discussion on whether molecular hydrogen can be evolved from this semiconductor. Our study on methanol photoreforming in the ultra-high vacuum reveals that photocatalysts comprising a TiO2(110) single crystal decorated with platinum clusters indeed enable the evolution of H2. This is attributed to a new type of mechanism, in which the co-catalyst acts as a recombination center for hydrogen and not as a reduction site of a photoreaction. This mechanism is an alternative pathway to the commonly used mechanism derived from photoelectrochemistry and must particularly be considered for systems, in which reducible semiconductors enable the surface diffusion of hydrogen species.


 Please wait while we load your content...
Please wait while we load your content...