An iron(ii) coordination polymer of a triazolyl tris-heterocycle showing a spin state conversion triggered by loss of lattice solvent†‡
Abstract
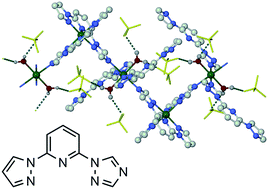
Solvated crystals of “[FeL2][BF4]2·H2O” (L = 2-(1,2,4-triazol-1-yl)-6-(pyrazol-1-yl)pyridine) contain a 1D coordination polymer, [Fe(μ-L)2{Fe(OH2)2L2}][BF4]4. Two iron environments linked by κ1:κ3,μ-L ligands alternate in the chains, Fe(1) being coordinated by two tridentate L ligands and Fe(2) by four monodentate L and two water molecules. Fe(1) is low-spin in a freshly prepared crystal at 150 K, but gradually converts to a high-spin form when that crystal was measured at higher temperatures. Magnetic data imply this is triggered by loss of ca. half a lattice water molecule during the experiment, rather being a thermal spin-crossover.


 Please wait while we load your content...
Please wait while we load your content...