Phase and morphology evolution of luminescent NaLnF4 (Ln = La to Yb) micro-crystals: understanding the ionic radii and surface energy-dependent solution growth mechanism†
Abstract
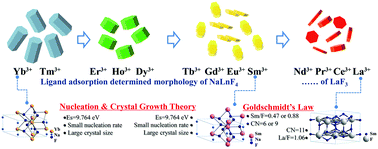
Lanthanide (Ln)-based NaLnF4 micro-crystals (MCs) exhibit great advantages and strong feasibility for integration into micron-sized photonic devices. However, there is still a lack of thermodynamic understanding of the solution growth mechanisms of NaLnF4 MCs. Herein, we report the preparation of hexagonal NaLnF4 MCs with various morphologies (rod-, disk- and flake-like) and crystal sizes (100 nm to 10 μm) via a hydrothermal route. The morphologies and sizes of NaLnF4 MCs were revealed to be highly dependent on the ionic radius of different Ln3+ ions. We have proposed a ligand-adsorption-driven oriented growth model and a surface energy dominant nucleation model to interpret the above phenomena. First, the citrate (Cit3−) adsorption on the {10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0} lattice plane primarily leads to different morphologies of NaLnF4 MCs. Second, different surface energies are required for nucleation and are responsible for crystal size evolution from NaYbF4 to LaF3. Third, alternative phase types from NaLnF4 to LnF3 are related to the Ln–F bond energy and Goldschmidt's law of crystal chemistry. In addition, the surface ligand removal is beneficial for the luminescence enhancement of NaLnF4 MCs. These results provide a thermodynamic understanding for researchers to develop NaLnF4 MCs with targeted morphology and size.
0} lattice plane primarily leads to different morphologies of NaLnF4 MCs. Second, different surface energies are required for nucleation and are responsible for crystal size evolution from NaYbF4 to LaF3. Third, alternative phase types from NaLnF4 to LnF3 are related to the Ln–F bond energy and Goldschmidt's law of crystal chemistry. In addition, the surface ligand removal is beneficial for the luminescence enhancement of NaLnF4 MCs. These results provide a thermodynamic understanding for researchers to develop NaLnF4 MCs with targeted morphology and size.


 Please wait while we load your content...
Please wait while we load your content...