Effects of vanadium pentoxide with different crystallinities on lithium ion storage performance†
Abstract
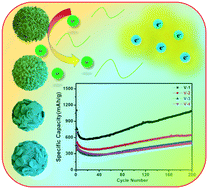
To investigate the effects of different crystallinities on the electrochemical performance of V2O5 as an anode material for lithium ion batteries, spherical V2O5 with variable crystallinities was prepared via a solvothermal method and changing the calcination temperature. The results confirmed that V2O5 with lower crystallinity showed a better lithium ion storage performance because of its loose structure, lower polarization, and the convenient diffusion of lithium ions. The reversible specific capacity of V2O5 obtained at 250 °C (1092.1 mA h g−1) with lower crystallinity was much higher than that of the others prepared at 350 °C (645.9 mA h g−1), 450 °C (526 mA h g−1), and 550 °C (498.9 mA h g−1) at 100 mA g−1 after 200 cycles. Also, it maintained a specific capacity of 584.6 mA h g−1 even at 1 A g−1 after 500 cycles. V2O5 prepared at 350 °C exhibited an excellent rate performance with a specific capacity of 131.1 mA h g−1 at a current density of 5 A g−1. The aim of this study was to propose an effective direction and strategy to design traditional metal oxide electrode materials with lower crystallinity to enhance the cycle and rate performance of lithium ion batteries in the future.


 Please wait while we load your content...
Please wait while we load your content...