Multi-stimuli responsive properties switch by intra- and inter-molecular charge transfer constructed from triphenylamine derivative†
Abstract
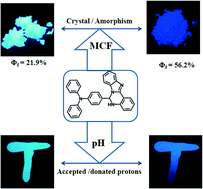
Combining triphenylamine (TPA) and 2-(1H-benzo[d]imidazol-2-yl) aniline (BI), a typical fluorescent dye, TPA-BI, was designed and synthesized. The photo-physical properties of this compound were investigated both in solution and the solid state. TPA-BI showed a strong AIEE effect and its relative fluorescence quantum yield (Φf) was up to 63.4% in the aggregation state. The tunable fluorescence phenomenon by the MCF effect was observed between the amorphous (bluish violet) and crystalline (green) states, and the absolute Φf was determined to be 56.2% and 21.9%, respectively, by the integral sphere method. Meanwhile, pH titration experiments exhibited the tunable fluorescence phenomenon in different pH solutions. Thus, TPA-BI could also be used as a fluorescent switch from green to bluish-violet based on its response to volatile acidic and alkaline organic compounds in the solid state repeatedly without fatigue and destruction under UV illumination (365 nm), which can be explained by the reversible transformation by protonation and deprotonation. The protonation process was further confirmed in detail by 1HNMR titration experiments. Density functional theory (DFT) and time-dependent density functional theory (TD-DFT) were used to predict the multi-stimuli responsive fluorescence properties.


 Please wait while we load your content...
Please wait while we load your content...