Crystal morphology prediction of energetic materials grown from solution: insights into the accurate calculation of attachment energies
Abstract
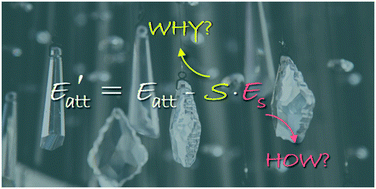
As a crucial factor, crystal morphology significantly influences the energy and safety properties of energetic materials (EMs). Theoretical prediction of crystal morphology is helpful for scientifically engineering the shape of EM crystals. Currently, the attachment energy (AE) model has been the most widely used method of crystal morphology prediction in the field of EMs. However, the calculation details of modified AE in solution and the physical meaning of some corrected parameters are still unclear, sometimes leading to discrepant predicted results even for the same EM. Here, HMX (1,3,5,7-tetranitro-1,3,5,7-tetrazocane) was taken as a case study and the details of AE calculation were systematically explored. The formula of AE in solution was corrected and a new functional form was proposed. The growth morphology of a HMX crystal in acetone solution was predicted, which is similar to those of the occupancy model, the spiral growth model, and the interfacial structure analysis model. The results reported in this contribution are aiming at the development of a more reasonable and accurate method for predicting the crystal shape of EMs.


 Please wait while we load your content...
Please wait while we load your content...