Photo-physical properties of salts of a di-topic imidazole-tethered anthracene derivative in solid and solution†
Abstract
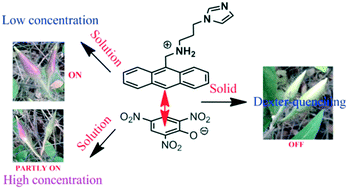
The overall effects of protonation and π-stacking influencing the photoluminescence of the di-topic 9-N-(3-imidazolylpropylamino)methylanthracene (HL) caused by mono-, di- and tri- nitrophenols, namely, 4-nitrophenol, 2,4-dinitrophenol and 2,4,6-trinitrophenol in solid and solution are presented. The observed photo-physical changes caused by nitrophenols are compared with those from 2-nitrobenzoic acid and 2,3-dihydroxybenzoic acid. Due to Dexter quenching, the solid sample of the 4-nitrophenolate salt is very weakly fluorescent, whereas the 2,4-dinitrophenolate and 2,4,6-trinitrophenolate salts are non-fluorescent. The emission of the 2-nitrobenzoate salt shows a 46 nm red-shift, whereas the 2,3-dihydroxybenzoate salt shows partial quenching without a shift. The flexible arm of the cation in each salt has different conformation adjustments, which are reflected in the respective packing patterns. The di-topic behavior of the HL is reflected in the fluorescence emission titrations performed in the solution state. At lower concentrations of nitrophenols, an increase in fluorescence is observed in each case, but at higher concentrations, quenching of the fluorescence emission of the HL is observed. However, upon the addition of 2-nitrobenzoic acid or 2,3-dihydroxybenzoic acid to a solution of HL, there is a slight increase in the emission intensity but no quenching takes place at higher concentrations.


 Please wait while we load your content...
Please wait while we load your content...