Visible light-induced palladium-catalyzed ring opening β-H elimination and addition of cyclobutanone oxime esters†
Abstract
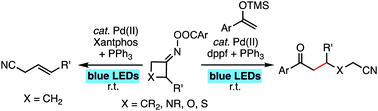
A palladium catalyst under visible light irradiation activates cyclobutanone oxime ester through single electron transfer to induce radical ring opening to generate hybrid cyanoalkyl Pd(I) radical species. Hybrid cyanoalkyl Pd(I) radical species can undergo either β-H elimination to deliver (E)-4-arylbut-3-enenitrile or undergo radical addition with silyl enol ether and enamide to generate δ-cyano ketones. A dual ligand system composed of two phosphine ligands is essential for the high reactivity.


 Please wait while we load your content...
Please wait while we load your content...