Asymmetric aerobic decarboxylative Povarov reactions of N-aryl α-amino acids with methylenephthalimidines via cooperative photoredox and chiral Brønsted acid catalysis†
Abstract
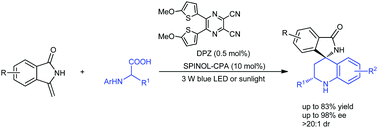
An enantioselective aerobic decarboxylative Povarov reaction of N-aryl α-amino acids with methylenephthalimidines through cooperative photoredox and chiral Brønsted acid catalysis is reported. With a transition metal-free dual catalytic system including a chiral phosphoric acid and DPZ as a photosensitizer mediated by visible light, the transformations provided a series of valuable chiral isoindolin-1-ones containing a 3,3-spiro-tetrahydroquinoline-based stereocenter in high yields (up to 83%) with good to excellent enantioselectivities (up to 98% ee) and excellent diastereoselectivity (>20 : 1 dr).


 Please wait while we load your content...
Please wait while we load your content...