Direct monitoring of equilibrium protein folding–unfolding by atomic force microscopy: pushing the limit†
Abstract
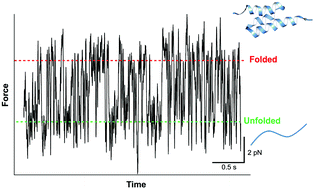
We report the direct observation of equilibrium folding–unfolding dynamics of a mechanically labile, three helix bundle protein GA using a commercial atomic force microscope (AFM). Our study extends the capability of commercial AFMs towards studying protein unfolding/refolding at equilibrium as well as increasingly complex biomolecular systems in the context of biological function.


 Please wait while we load your content...
Please wait while we load your content...