Chirality transfer in a cage controls the clockwise/anticlockwise propeller arrangement of the tris(2-pyridylmethyl)amine ligand†
Abstract
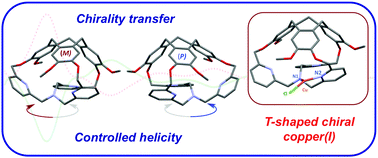
Predictable control of the propeller arrangement of the tris(2-pyridyl-methyl)amine (TPA) ligand was achieved through the preparation of the smallest hemicryptophane 1. This newly designed cage displays a chirality transfer from its northern unit to the TPA ligand. 1 can coordinate Cu(I), yielding a rare T-shaped complex with controlled helicity of the TPA-Cu(I) core.


 Please wait while we load your content...
Please wait while we load your content...