Catalyst-free phosphorylation of aryl halides with trialkyl phosphites through electrochemical reduction†
Abstract
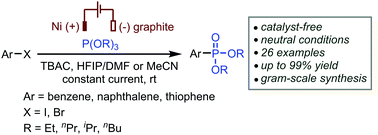
A catalyst-free electrochemical cross-coupling reaction of aryl halides with trialkyl phosphite has been developed. This reaction proceeds in an undivided cell with a low-cost Ni anode and a graphite cathode under mild and neutral conditions. A wide range of functional groups are well-tolerated and the phosphorylated product can be obtained on the gram scale, showing that this transformation has the potential to be a valuable method for the construction of aromatic carbon–phosphorus bonds.


 Please wait while we load your content...
Please wait while we load your content...