Arylallenes and the halogeno-B(C6F5)2 reagents: facile formation of 2-borylindenes†
Abstract
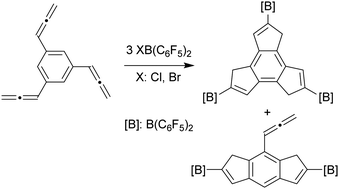
Phenylallene reacts rapidly with ClB(C6F5)2 to give the respective 2-borylindene. Several substituted allenylarenes form the respective 2-B(C6F5)2 boryl-substituted indenes upon treatment with ClB(C6F5)2 or BrB(C6F5)2 as well. Bis- and tris-allenylarenes form the corresponding products featuring multiple five-membered ring annulations, including a symmetrical tris-borylated dihydro-1H-trindene derivative. The B(C6F5)2 borylindenes show fluorescence properties.


 Please wait while we load your content...
Please wait while we load your content...