Synthesis and electronic properties of π-expanded carbazole-based porphyrins†
Abstract
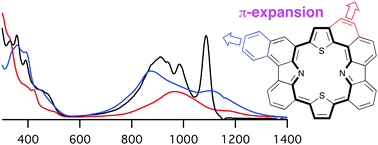
Peripheral π-expansion of carbazole-based porphyrins was achieved for the first time by the Pt-catalyzed cyclization and the incorporation of a benzocarbazole unit. These two types of fused porphyrins showed red-shifted and broad NIR absorption due to the expanded π-conjugation as confirmed by NIR absorption spectroscopy and DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...