Bifunctional electrocatalysis for CO2 reduction via surface capping-dependent metal–oxide interactions†
Abstract
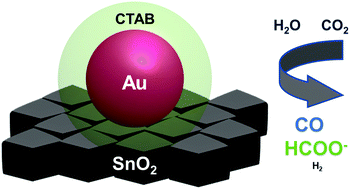
Multi-component materials are a new trend in catalyst development for electrochemical CO2 reduction. Understanding and managing the chemical interactions within a complex catalyst structure may unlock new or improved reactivity, but is scientifically challenging. We report the first example of capping ligand-dependent metal–oxide interactions in Au/SnO2 structures for electrocatalytic CO2 reduction. Cetyltrimethylammonium bromide capping on the Au nanoparticles enables bifunctional CO2 reduction where CO is produced at more positive potentials and HCOO− at more negative potentials. With citrate capping or no capping, the Au–SnO2 interactions steer the selectivity toward H2 evolution at all potentials. Using electrochemical CO oxidation as a probe reaction, we further confirm that the metal–oxide interactions are strongly influenced by the capping ligand.
- This article is part of the themed collection: 2019 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...