Functionalisation of isoindolinones via a calcium catalysed Hosomi–Sakurai allylation†
Abstract
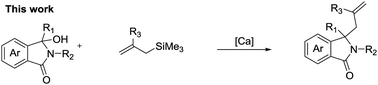
A rapid and functionally tolerant calcium catalysed Hosomi–Sakurai reaction has been realised. Employing 1 mol% calcium, allylated isoindolinones can be synthesised in high yields and the reaction is shown to be tolerant to a range of medicinally relevant functional groups including heterocycles. The synthetic utility of the reaction has been shown, and a plausible reaction mechanism is provided.


 Please wait while we load your content...
Please wait while we load your content...