Molecular doping: accessing the first carborane-substituted 1,2,3-triphospholanide via insertion of P− into a P−P bond†
Abstract
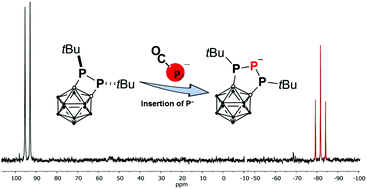
Insertion of a P− anion into a P–P bond yielding the first carborane-substituted 1,2,3-triphospholanide 1 was achieved by treating a carborane-substitued 1,2-diphosphetane with sodium phosphaethynolate. The triphospholanide 1 can serve as a versatile nucleophilic building block for unprecedent functionalised triphospholanes and carborane-substituted polyphosphanes.


 Please wait while we load your content...
Please wait while we load your content...