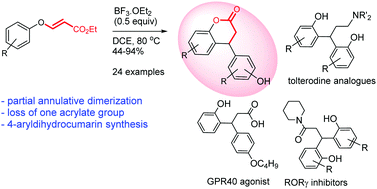
Lewis acid-catalyzed annulative partial dimerization of 3-aryloxyacrylates to 4-arylchroman-2-ones: synthesis of analogues of tolterodine, RORγ inhibitors and a GPR40 agonist†
Abstract
A beguiling annulative partial dimerization of 3-aryloxyacrylates to 4-arylchroman-2-ones catalyzed by Lewis acid (BF3·OEt2) has been developed. The reaction involves two molecules of 3-aryloxyacrylate, resulting in the loss of one propiolate molecule to furnish 4-arylchroman-2-one, an important structural motif found in many natural products. This methodology has been elaborated to synthesize analogues of tolterodine, RORγ inhibitors and a GPR40 agonist.


 Please wait while we load your content...
Please wait while we load your content...