Methanesulfonyl-polarized halogen bonding enables strong halide recognition in an arylethynyl anion receptor†
Abstract
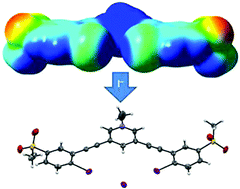
A 3,5-bis((2-iodophenyl)ethynyl)pyridinium scaffold was synthesized which introduces the use of methanesulfonyl withdrawing groups to polarize iodine halogen bonding units for anion binding. We investigate the capability of this receptor to bind halides in polar media, while further probing the structure–property relationship of this well-polarized yet under-explored halogen bonding system.


 Please wait while we load your content...
Please wait while we load your content...