A one-pot, water compatible synthesis of pyrimidine nucleobases under plausible prebiotic conditions†
Abstract
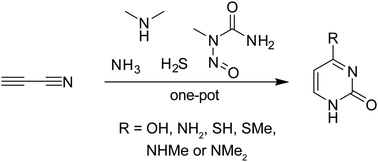
Herein, we report a new prebiotically plausible pathway towards a pyrimidine nucleobase in continuous manner. The route involves simultaneous methylation and carbamoylation of cyanoacetylene-derived α,β-unsaturated thioamide with N-methyl-N-nitrosourea (MNU) in aqueous media. This provides S-methylpyrimidinone in one-pot, which can be converted into a variety of 4-substituted pyrimidine nucleobases including cytosine and uracil.


 Please wait while we load your content...
Please wait while we load your content...