An organic-base catalyzed asymmetric 1,4-addition of tritylthiol to in situ generated aza-o-quinone methides at the H2O/DCM interface†
Abstract
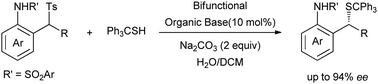
Based on an efficient method for in situ generation of N-o-QM species in the presence of a base, enantioselective catalytic conjugated additions of tritylthiol to in situ generated N-o-QMs are reported. Acid–base bifunctional organocatalyst 4c (10 mol%) enables these transformations with high stereoselectivities (up to 94% ee) using H2O/DCM as a solvent under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...