Affinity analysis between trypsin and aptamers using surface plasmon resonance competition experiments in a steady state
Abstract
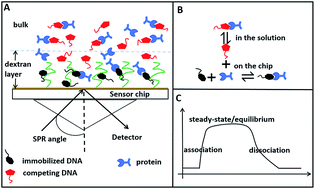
A surface plasmon resonance (SPR) competition experiment in a steady state was developed to determine the binding dissociation constants between a protein and its DNA aptamers. The affinities of a large set of trypsin aptamers selected by magnetic beads-systematic evolution of ligands by exponential enrichment (MB-SELEX) and capillary electrophoresis (CE-SELEX) are obtained only on one single chip. A large number of chips and a considerable amount of time are saved compared with a typical SPR experiment. Additionally, this approach does not require prior knowledge of parameters, such as on or off rates, using a nonlinear fitting with a known dissociation constant and the protein concentration as input. Knowledge on the specificity of protein–aptamer interaction is also obtained by the SPR competition experiment.

 Please wait while we load your content...
Please wait while we load your content...