Highly selective hydrogenation of amides catalysed by a molybdenum pincer complex: scope and mechanism†
Abstract
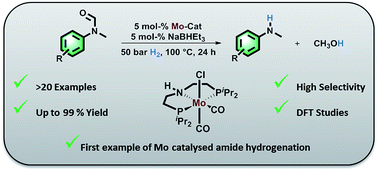
A series of molybdenum pincer complexes has been shown for the first time to be active in the catalytic hydrogenation of amides. Among the tested catalysts, Mo-1a proved to be particularly well suited for the selective C–N hydrogenolysis of N-methylated formanilides. Notably, high chemoselectivity was observed in the presence of certain reducible groups including even other amides. The general catalytic performance as well as selectivity issues could be rationalized taking an anionic Mo(0) as the active species. The interplay between the amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O reduction and the catalyst poisoning by primary amides accounts for the selective hydrogenation of N-methylated formanilides. The catalyst resting state was found to be a Mo–alkoxo complex formed by reaction with the alcohol product. This species plays two opposed roles – it facilitates the protolytic cleavage of the C–N bond but it encumbers the activation of hydrogen.
O reduction and the catalyst poisoning by primary amides accounts for the selective hydrogenation of N-methylated formanilides. The catalyst resting state was found to be a Mo–alkoxo complex formed by reaction with the alcohol product. This species plays two opposed roles – it facilitates the protolytic cleavage of the C–N bond but it encumbers the activation of hydrogen.


 Please wait while we load your content...
Please wait while we load your content...