Engineering an NIR rhodol derivative with spirocyclic ring-opening activation for high-contrast photoacoustic imaging†
Abstract
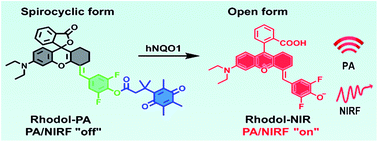
Molecular probes that enable high-contrast photoacoustic (PA) imaging of cellular processes are valuable tools for in vivo studies. Design of activatable PA probes with high contrast remains elusive. We develop a new NIR rhodol derivative, Rhodol-NIR, with a large extinction coefficient, low quantum yield and structural switching from a ‘ring-open’ form to a ‘closed’ spirolactone upon esterification. This structural transition, together with the ideal photophysical properties, enables the development of activatable probes for high-contrast PA imaging via a target-specific de-esterification reaction. This strategy is demonstrated using a PA probe designed for a tumor biomarker, human NAD(P)H: quinone oxidoreductase isozyme 1 (hNQO1), which affords high contrast and excellent sensitivity for PA detection and imaging of hNQO1 in living cells and animals. The strategy can provide a new paradigm for engineering activatable PA probes for high-contrast imaging.


 Please wait while we load your content...
Please wait while we load your content...