Saturated oxygen and nitrogen heterocycles via oxidative coupling of alkyltrifluoroborates with alkenols, alkenoic acids and protected alkenylamines†
Abstract
Saturated heterocycles are important components of many bioactive compounds. The method disclosed herein enables a general route to a range of 5-, 6- and 7-membered oxygen and nitrogen heterocycles by coupling potassium alkyltrifluoroborates with heteroatom-tethered alkenes, predominantly styrenes, under copper-catalyzed conditions, in the presence of MnO2. The method was applied to the synthesis of the core of the anti-depressant drug citalopram. The reaction scope and observed reactivity is consistent with a polar/radical mechanism involving intermolecular addition of the alkyl radical to the alkene followed by [Cu(III)]-facilitated C–O (or C–N) bond forming reductive elimination.
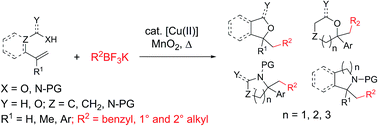
- This article is part of the themed collections: Editor’s Choice – Ning Jiao and New reactivity in organic chemistry


 Please wait while we load your content...
Please wait while we load your content...