Near-infrared fluorescein dyes containing a tricoordinate boron atom†
Abstract
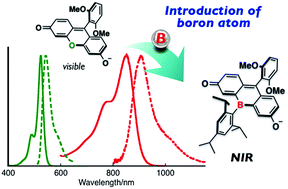
Bora-fluoresceins (BFs), fluorescein analogues containing a tricoordinate boron atom instead of an oxygen atom at the 10-position of the fluorescein skeleton, were synthesized as a new family of fluorescein analogues. The deprotonated BFs exhibited absorption and fluorescence in the near-infrared region, which were significantly red-shifted relative to those of hitherto-known heteroatom-substituted fluorescein analogues on account of the orbital interaction between the tricoordinate boron atom and the fluorescein skeleton. BFs also showed multi-stage changes resulting from a Lewis acid–base equilibrium at the boron center in combination with a Brønsted acid–base equilibrium at the phenolic hydroxy group.
- This article is part of the themed collection: Near-infrared (NIR) luminescent probes for bioimaging and biosensing


 Please wait while we load your content...
Please wait while we load your content...