Tuning ligand field strength with pendent Lewis acids: access to high spin iron hydrides†
Abstract
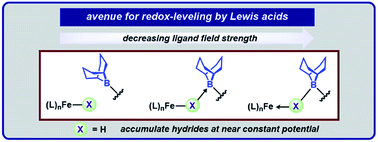
Geometrically flexible 9-borabicyclo[3.3.1]nonyl units within the secondary coordination sphere enable isolation of high-spin Fe(II)-dihydrides stabilized by boron–hydride interactions and a rare example of an isolable S = 3/2 reduction product. The borane-capped Fe(II)-dihydride: (1) rapidly deprotonates E–H (E = N, O, P, S) bonds to afford borane-stabilized Fe adducts and (2) releases H2 upon exposure to π-acids. The Lewis acids provide an avenue for redox-leveling in analogy to the near constant operating potential for N2 reduction in nitrogenase.
- This article is part of the themed collections: Spotlighting main group elements in polynuclear complexes and Editor's Choice – Serena DeBeer


 Please wait while we load your content...
Please wait while we load your content...