Leoligin-inspired synthetic lignans with selectivity for cell-type and bioactivity relevant for cardiovascular disease†‡
Abstract
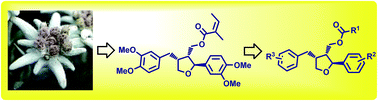
Recently, a natural compound leoligin, a furan-type lignan, was discovered as an interesting hit compound with an anti-inflammatory pharmacological activity profile. We developed a modular and stereoselective approach for the synthesis of the edelweiss-derived lignan leoligin and used the synthetic route to rapidly prepare leoligin analogs even on the gram scale. Proof of concept of this approach together with cell-based bio-assays gained structural analogs with increased selectivity towards vascular smooth muscle versus endothelial cell proliferation inhibition, a major benefit in fighting vascular neointima formation. In addition, we identified the structural features of leoligin analogs that define their ability to inhibit the pro-inflammatory NF-κB pathway. Results are discussed in the context of structural modification of these novel synthetic lignans.


 Please wait while we load your content...
Please wait while we load your content...