Hydroxyl-mediated ethanol selectivity of CO2 hydrogenation†
Abstract
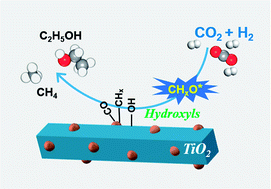
Oxide-supported Rh nanoparticles have been widely used for CO2 hydrogenation, especially for ethanol synthesis. However, this reaction operates under high pressure, up to 8 MPa, and suffers from low CO2 conversion and alcohol selectivity. This paper describes the crucial role of hydroxyl groups bound on Rh-based catalysts supported on TiO2 nanorods (NRs). The RhFeLi/TiO2 NR catalyst shows superior reactivity (≈15% conversion) and ethanol selectivity (32%) for CO2 hydrogenation. The promoting effect can be attributed to the synergism of high Rh dispersion and high-density hydroxyl groups on TiO2 NRs. Hydroxyls are proven to stabilize formate species and protonate methanol, which is easily dissociated into *CHx, and then CO obtained from the reverse water–gas shift reaction (RWGS) is inserted into *CHx to form CH3CO*, followed by CH3CO* hydrogenation to ethanol.
- This article is part of the themed collections: Celebrating the Chemical Sciences in India - Leaders in the Field Symposium 2021 and 2019 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...