Making the unconventional μ2-P bridging binding mode more conventional in phosphinine complexes†
Abstract
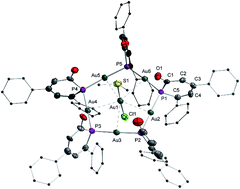
Phosphinines, as aromatic heterocycles, usually engage in coordination as η1-P σ-complexes or η6-phosphinine π-complexes. The μ2-P bridging coordination mode is rarely observed. With the aim to study the effect of different electronic configurations of phosphinines on the coordination modes, a series of anionic phosphinin-2-olates and neutral phosphinin-2-ols were prepared with moderate to high yield. Then the coordination chemistry of these two series was studied in detail towards coinage metals (Au(I) and Cu(I)). It is observed that the anionic phosphinin-2-olates possess a higher tendency to take a bridging position between two metal centers compared to the neutral phosphinin-2-ols. Based on these experimental findings bolstered by DFT calculations, some insight is gained on how the unconventional μ2-P phosphinine bridging coordination mode can be made more conventional and used for the synthesis of polynuclear complexes.


 Please wait while we load your content...
Please wait while we load your content...