Connective synthesis of 5,5-disubstituted hydantoins by tandem α-amination and α-arylation of silyl ketene acetals†
Abstract
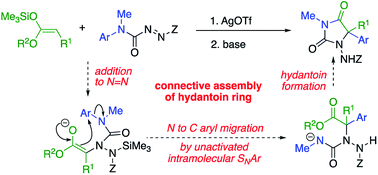
5,5-Disubstituted hydantoins, formally the cyclisation products of quaternary amino acids, were formed connectively from simple ester-derived starting materials by a one-pot tandem method. Amination of the silyl ketene acetal derivative of a methyl ester takes place by silver-catalysed addition to the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of an azocarboxamide, generating a N-amino-N′-aryl urea derivative of a substituted aminoester. Treatment with a base forms an ester enolate which undergoes arylation by intramolecular migration of an aryl ring to the α-position of the ester. The product undergoes ring closure to a hydantoin, which may itself be deprotected and functionalised. Aryl migration is successful with rings of various electronic character and with esters bearing functionalised and unfunctionalised chains, and the products have features in common with several bioactive compounds.
N bond of an azocarboxamide, generating a N-amino-N′-aryl urea derivative of a substituted aminoester. Treatment with a base forms an ester enolate which undergoes arylation by intramolecular migration of an aryl ring to the α-position of the ester. The product undergoes ring closure to a hydantoin, which may itself be deprotected and functionalised. Aryl migration is successful with rings of various electronic character and with esters bearing functionalised and unfunctionalised chains, and the products have features in common with several bioactive compounds.


 Please wait while we load your content...
Please wait while we load your content...