Primary α-tertiary amine synthesis via α-C–H functionalization†
Abstract
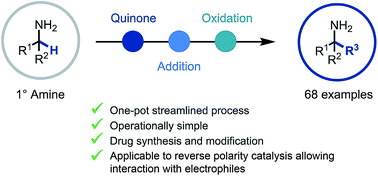
A quinone-mediated general synthetic platform for the construction of primary α-tertiary amines from abundant primary α-branched amine starting materials is described. This procedure pivots on the efficient in situ generation of reactive ketimine intermediates and subsequent reaction with carbon-centered nucleophiles such as organomagnesium and organolithium reagents, and TMSCN, creating quaternary centers. Furthermore, extension to reverse polarity photoredox catalysis enables reactivity with electrophiles, via a nucleophilic α-amino radical intermediate. This efficient, broadly applicable and scalable amine-to-amine synthetic platform was successfully applied to library and API synthesis and in the functionalization of drug molecules.
- This article is part of the themed collections: Most popular 2019-2020 organic chemistry articles, Most popular 2018-2019 organic chemistry articles and The ChemRxiv Collection


 Please wait while we load your content...
Please wait while we load your content...