Enhanced catalytic activity of inhomogeneous Rh-based solid-solution alloy nanoparticles†
Abstract
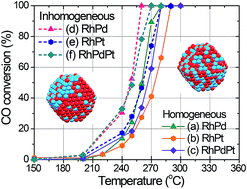
Catalytic Rh-based alloy nanoparticles (NPs) with inhomogeneous solid-solution structures were prepared from homogeneous solid-solution alloy NPs. Compared with homogeneous alloy NPs, these inhomogeneous alloy NPs exhibited enhanced catalytic activity and superior catalytic durability. Homogeneous solid-solution alloy NPs consisting of Rh and other immiscible noble metals were synthesized by laser-induced nucleation method in metallic ion solutions. STEM elemental mapping and EDS composition analysis of the particles clearly demonstrated that all the constituents were uniformly dispersed within the NPs. Moreover, the compositions of the alloys were nearly identical to the initial feeding ratios of metallic ions in the mixed solutions, strongly indicating the formation of equimolar solid-solution alloy NPs over the entire composition range. Although the catalytic stability of these Rh-based homogeneous alloy NPs during CO oxidation was improved, their catalytic activity was comparable to that of pure metal catalysts, owing to the uniform local structure at the atomic level. However, the catalytic activity of the alloy NPs was enhanced by heat treatment, which introduced inhomogeneity in the atomic distribution within the NPs. The enhanced activity was due to dissimilar interfaces in the inhomogeneous solid-solution alloy NPs.


 Please wait while we load your content...
Please wait while we load your content...