Efficient catalytic oxidation of methyl aromatic hydrocarbon with N-alkyl pyridinium salts†
Abstract
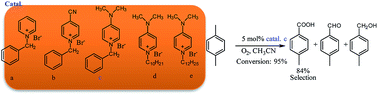
A series of N-alkyl pyridinium salts were synthesized and employed as metal-free catalyst for the selective oxidation of methyl aromatic hydrocarbon with molecular oxygen. The electronic effect of the substitutes was found to be an important factor for the catalytic performance. With the introduction of electron-donating substitute –N(CH3)2, the conversion of p-xylene and selectivity of p-toluic acid could be simultaneously increased. 1-Benzyl-4-N,N-dimethylaminopyridinium salt showed the highest catalytic activity, and 95% conversion with 84% of selectivity to p-toluic acid could be obtained for the selective oxidation of p-xylene. Several methyl aromatic hydrocarbons could all be efficiently oxidized with the reported catalyst at the absence of any metal species.


 Please wait while we load your content...
Please wait while we load your content...