A novel chiral DMAP–thiourea bifunctional catalyst catalyzed enantioselective Steglich and Black rearrangement reactions†
Abstract
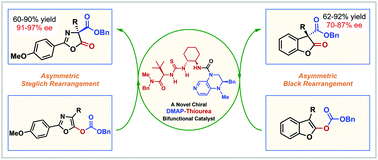
The first novel and efficient chiral DMAP–thiourea bifunctional catalyst has been successfully developed and applied in highly enantioselective acyl-transfer reactions. A series of 1,3-oxazolyl carbonates are efficiently transformed to C-carboxyazlactones (Steglich rearrangement) by employing 5 mol% of this bifunctional nucleophilic catalyst with good yields and excellent enantioselectivities (up to 90% yield and 97% ee), and 2-benzofuranylcarbonates are also converted to 3,3-disubstituted benzofuran-2-ones (Black rearrangement) with satisfactory asymmetric induction (up to 92% yield and 87% ee).
- This article is part of the themed collection: 2019 Organic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...