Ligandless nickel-catalyzed transfer hydrogenation of alkenes and alkynes using water as the hydrogen donor†
Abstract
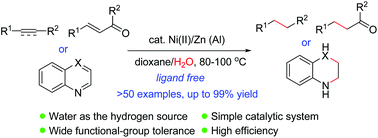
The first general route for nickel-catalyzed transfer hydrogenation reaction of alkenes and alkynes using water as the hydrogen source has been developed. The method features the use of inexpensive and air-stable nickel(II) salt as the pre-catalyst and zinc powder as a reducing agent, allowing the TH reaction to occur under mild reaction conditions with a wide substrate scope and functional group tolerance. No ligand was required for this reaction. The reaction has also been applied successfully to the reduction of nitrogen-containing heterocycles.
- This article is part of the themed collections: Celebrating 70 Years of Shanghai Institute of Organic Chemistry and 2019 Organic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...