Impact of building block structure on ion transport in cyclopropenium-based polymerized ionic liquids†
Abstract
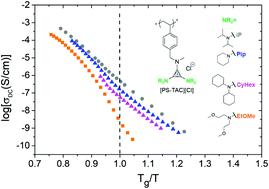
Ion transport and morphology are studied in cationic polymers based on tris(dialkyl)aminocyclopropenium ions tethered onto polystyrene (PS-TAC) and Cl− counterions. To investigate how molecular structure impacts single-ion transport and physical properties, the substituents on TAC were varied. Modifying the polarity, along with the size and flexibility of the functional groups on TAC significantly changes the glass transition temperature. Ionic conductivity of the PS-TAC with branched functional groups is 1–2 orders of magnitude lower than more compact and bulkier moieties on polymers at their glass transition temperature, Tg. Decreasing the size of the substituents correlates with increasing ionic conductivity in the polymers with non-polar functional groups. However, it is the geometry of the functional groups on the cation (isopropyl, ring, or linear) in PS-TAC that has a much larger effect on conductivity than the size of the group itself. Mimicking the transport properties of ionic liquids using polymers imbued with mechanical stability is essential for the development of robust, non-volatile electrolytes for batteries and fuel cells. Changing a number of variables in this tunable PS-TAC system is a step towards developing soft materials design rules for selecting solid ion supports.


 Please wait while we load your content...
Please wait while we load your content...