HClO4 catalysed aldol-type reaction of fluorinated silyl enol ethers with acetals or ketals toward fluoroalkyl ethers†
Abstract
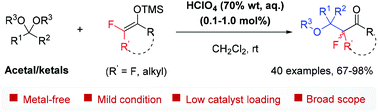
A highly efficient metal-free aldol-type reaction of various acetals or ketals with fluorinated silyl enol ethers catalysed by less than 1 mol% HClO4 (70 wt%, aq.) is developed. This provides expedient access to a wide array of valuable fluoroalkyl ethers featuring a ketone carbonyl functionality in good to excellent yields (40 examples). Furthermore, the thus obtained adducts are readily elaborated into other fluorine-containing alkyl ethers with a rich structure.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...