Cu-Mediated arylselenylation of aryl halides with trifluoromethyl aryl selenonium ylides†
Abstract
An unprecedented arylselenylation of aryl halides with trifluoromethyl aryl selenonium ylides in the presence of copper is described. The reaction proceeded at 100–140 °C under ligand- and additive-free conditions for 3–20 h to form a variety of unsymmetrical diaryl selenides in good to high yields. Arylselenylation is easy to operate, has good functional group tolerance, and demonstrates the different reaction profiles of trifluoromethyl aryl selenonium ylides from the homologous trifluoromethyl aryl sulfonium ylides.
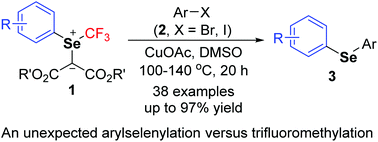
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...