An efficient tandem synthesis of chromones from o-bromoaryl ynones and benzaldehyde oxime†
Abstract
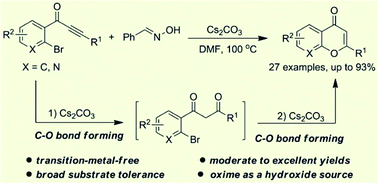
An effective transition-metal-free strategy was developed for the preparation of chromones from o-bromoaryl ynones and benzaldehyde oxime through sequential C–O bond formation. This cyclization reaction could well tolerate a wide range of functional groups, and the corresponding chromones were given in moderate to excellent yields. Mechanistically, benzaldehyde oxime as a hydroxide source and 1,3-diketone derivatives as reaction intermediates were involved in this transformation.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...