Metal-free difunctionalization of alkynes to access tetrasubstituted olefins through spontaneous selenosulfonylation of vinylidene ortho-quinone methide (VQM)†
Abstract
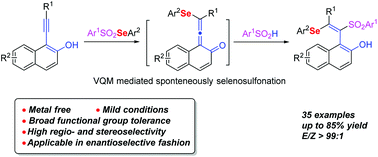
A metal-free difunctionalization of alkynes to access tetrasubstituted olefins through spontaneous selenosulfonylation of vinylidene ortho-quinone methide (VQM) was described herein. The reaction was conducted under mild conditions without any catalysts or additives. Preliminary mechanism studies revealed that the formation of VQM was the key for this alkyne di-functionalization reaction. The reaction could be applied in the enantioselective asymmetric synthesis of axially chiral styrene. Furthermore, the selenosulfonylation adducts can be transformed into useful naphtho[2,1-b]furan and benzofuran scaffolds.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...