The protosteryl and dammarenyl cation dichotomy in polycyclic triterpene biosynthesis revisited: has this ‘rule’ finally been broken?†
Abstract
Covering: 1948 up to the end of 2018.
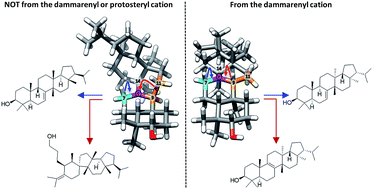
The triterpene alcohols represent an important and diverse class of natural products. This diversity is believed to originate from the differential enzymatically controlled cyclisation of 2,3-oxidosqualene. It is now a well-established presumption that all naturally occurring tetra- and penta-cyclic triterpene alcohols can be rationalised by the resolution of one of two intermediary tetracyclic cations, termed the protosteryl and dammarenyl cations. Here, a discussion of typical key triterpene structures and their proposed derivation from either of these progenitors is followed by comparison with a recently reported novel pentacyclic triterpene orysatinol which appears to correspond to an unprecedented divergence from this dichotomous protosteryl/dammarenyl view of triterpene biogenesis. Not only does this discovery widen the potential scope of triterpene scaffolds that could exist in nature, it could call into question the reliability of stereochemical assignments of some existing triterpene structures that are supported by only limited spectroscopic evidence. The discovery of orysatinol provides direct experimental evidence to support considering more flexibility in the stereochemical interpretation of the biogenic isoprene rule.


 Please wait while we load your content...
Please wait while we load your content...