Low-temperature activation of carbon black by selective photocatalytic oxidation†
Abstract
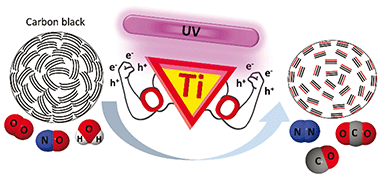
Carbon black is chemically modified by selective photocatalytic oxidation, removing amorphous carbon and functionalizing the graphitic fraction to produce porous, graphitized carbon black, commonly used as an adsorbent in chromatography. In contrast to pyrolytic treatments, this photocatalytic modification proceeds under mild reaction conditions using oxygen, nitric oxide, water vapor and a titanium dioxide photocatalyst at 150 °C. The photo-oxidation can be performed both with the photocatalyst in close proximity (contact mode) or physically separated from the carbon. Structural analysis of remotely photo-oxidized carbon black reveals increased hydrophilic properties as compared to pyrolysis at 700 °C in a N2 atmosphere. Carbon black photo-oxidation selectively mineralizes sp3-hybridized carbon, leading to enhanced graphitization. This results in an overall improved structural ordering by enriching carbon black with sp2-hybridized graphitic carbon showing decreased interplanar distance, accompanied by a twofold increase in the specific surface area. In addition, the photo-oxidized material is activated by the presence of oxygen functionalities on the graphitic carbon fraction, further enhancing the adsorptive properties.
- This article is part of the themed collection: Photocatalysis and Photoelectrochemistry


 Please wait while we load your content...
Please wait while we load your content...