Kinetics and mechanism for hydrothermal conversion of polyhydroxybutyrate (PHB) for wastewater valorization†
Abstract
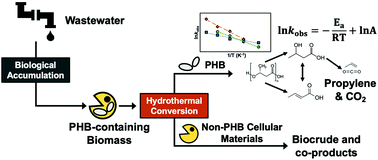
Conventional wastewater treatment processes can be tailored to recover organic carbon from wastewater as intracellular polyhydroxybutyrate (PHB) polymer granules while simultaneously meeting effluent discharge standards. Traditional applications of PHB as a bioplastic are hampered by its suboptimal properties (e.g., brittle), lack of efficient and sustainable approaches for recovering PHB from cells, and concerns about wastewater-derived impurities. In this study, we report on the conversion of PHB and its monomer acids – 3-hydroxybutyric acid (3HBA) and crotonic acid (CA) – under hydrothermal conditions (in condensed water at elevated temperature and pressure) to form propylene, a valuable chemical intermediate that self-separates from water. PHB depolymerization results in a mixture of 3HBA and CA, which can interconvert via (de)hydration reactions that vary with prevailing reaction conditions. Further hydrothermal conversion of the monomer acids yields propylene and CO2. Conversion of 3HBA occurs at lower temperatures than CA, and a new concerted dehydration-decarboxylation pathway is proposed, which differs from the sequential dehydration (3HBA to CA) and decarboxylation (CA to propylene and CO2) pathway reported for dry thermal conversion. A kinetics network model informed by experimental results reveals that CA conversion to propylene and CO2 proceeds predominantly via hydration to 3HBA followed by the concerted dehydration-decarboxylation pathway rather than by direct decarboxylation of CA. Demonstrative experiments using PHB-containing methanotrophic biomass show results consistent with the model, producing propylene at near-theoretical yields at lower temperatures than reported previously.


 Please wait while we load your content...
Please wait while we load your content...